WuXi Biologics
Offering End-to-End Solutions
Viral Vaccines Process Development
Leveraging WuXi Biologics’ HEK 293 Cells for Optimal Performance
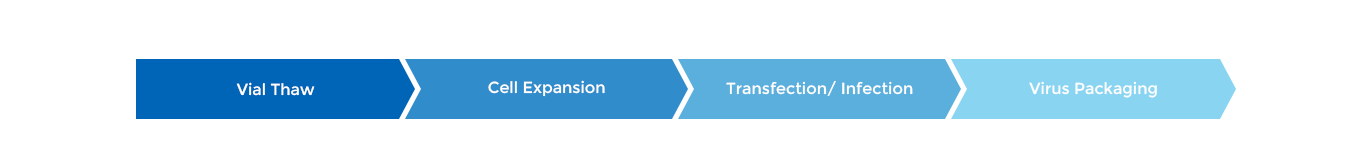
Viral Vaccine Cell Culture Process Development
- Multiple cell culture modes
-
- Batch, fed-batch or perfusion
- Flexible cell culture format
-
- Adherent cell culture (Cell Factory / cellSTAK, iCellis fixed-bed bioreactor) and microcarrier-based adherent cell culture
- Suspension cell culture (Bioreactor)
- Commercial media and chemically defined media preferred
Virus Purification Process Development
- Early stage feasibility study
- Process development towards IND filing
- Late stage process geared-characterization

Innovations To Increase Virus Titer
| Suspension Cell Culture |
|
|
Adherent Cell Culture
|
|
Notice:
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?





