WuXi Biologics
Offering End-to-End Solutions
Microbial Services Development
Beginning from strain development to CMC dossier and regulatory support, our comprehensive, end-to-end microbial production system harnesses highly trained staff and world-class quality systems, which have continually passed multiple rigorous rounds of regulatory agency inspections. We provide expert, efficient development services for vaccines produced via microbial fermentation, regardless of whether your product is nucleic acid- or protein-based. Explore more details of our single-source service offering.
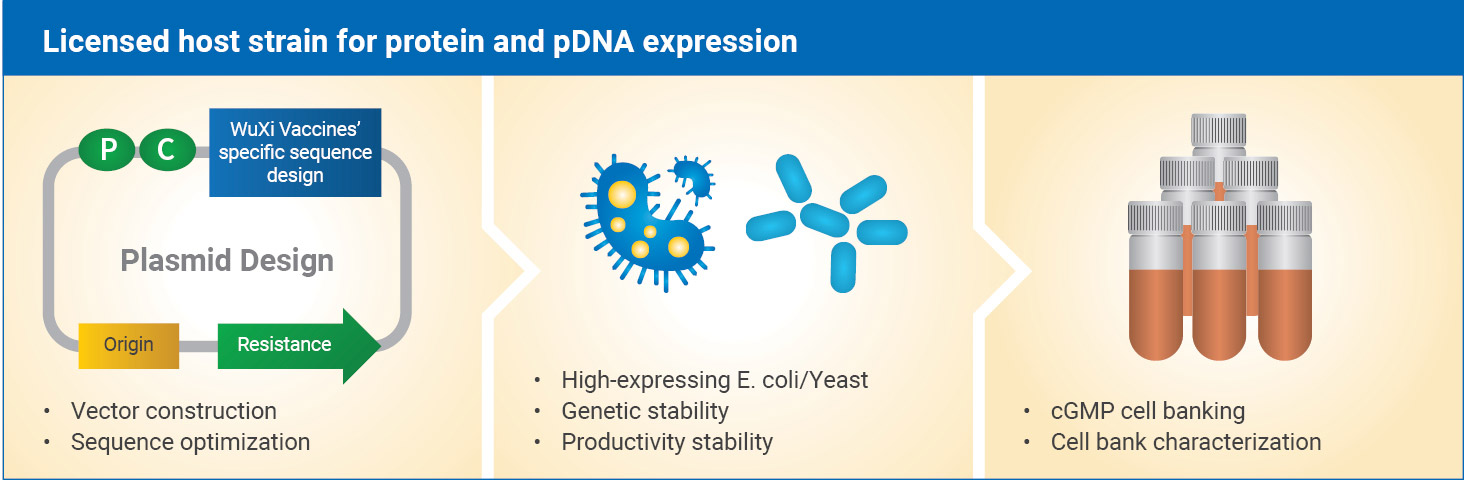
Strain Development
Strain development capabilities include:
- E. coli and yeast host systems
- Expression vector construction
- Sequence and codon optimization and signal peptide screening
- Clone screening
- cGMP cell banking and release testing
Drug Substance (DS) Process Development
Led by a team of experts, we offer batch and fed-batch, and downstream purification process development of molecules in different expression systems. This includes, but is not limited to, intracellular soluble products, inclusion bodies, and extracellular secreted products. Our multi-purpose/multi-product 2,000 m2 process development laboratories facilitate the development of nucleic acid- or protein-based vaccines, supporting IND-enabling toxicology studies, or preclinical and CMC development activities at various scales.
Our capabilities include:
- Strain/clone selection support
- Fermentation process development
- Purification process development
- Process verification for direct technical transfer
- Non-GMP and pilot production
- Scale-up, technical transfer and clinical manufacturing support
- Process characterization, optimization and validation
- IND/IMPD & BLA filing support
Formulation and Drug Product Development
We provide a dedicated team of highly-trained and experienced scientists capable of developing and optimizing vaccine product formulations filled into a wide variety of container and closure systems (CCS) including combination products (e.g., prefilled syringes and autoinjectors).
Our capabilities include:
- Labs with processing and physicochemical testing ability
- Pre-formulation and formulation development
- Liquid, frozen liquid and lyophilized dosage form development
- Forced degradation studies
- Container closure selection/evaluation including vials and prefilled syringes (PFS), PFS with needle safety devices (NSD) and autoinjectors (AI)
- Formulation of complex delivery systems including adjuvanted multivalent antigens, VLPs, lipid nanoparticles, and emulsions
- Clinical in-use compatibility
- Adjuvant selection and evaluation
- Process scale-up, technology transfer and process performance qualification
Analytical Development
The analytical method development team supports many CMC activities including:
- Development of in-process, release and stability-indicating methods
- Product characterization (biochemical, biophysical, biological); comparability assessment
- Forensic and analytical investigation; troubleshooting of GMP manufacturing-related investigations
- Lot release and stability studies of non-GMP batches
- Reference standard generation and characterization
- Technology transfer to quality control (QC)
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






