WuXi Biologics
Offering End-to-End Solutions
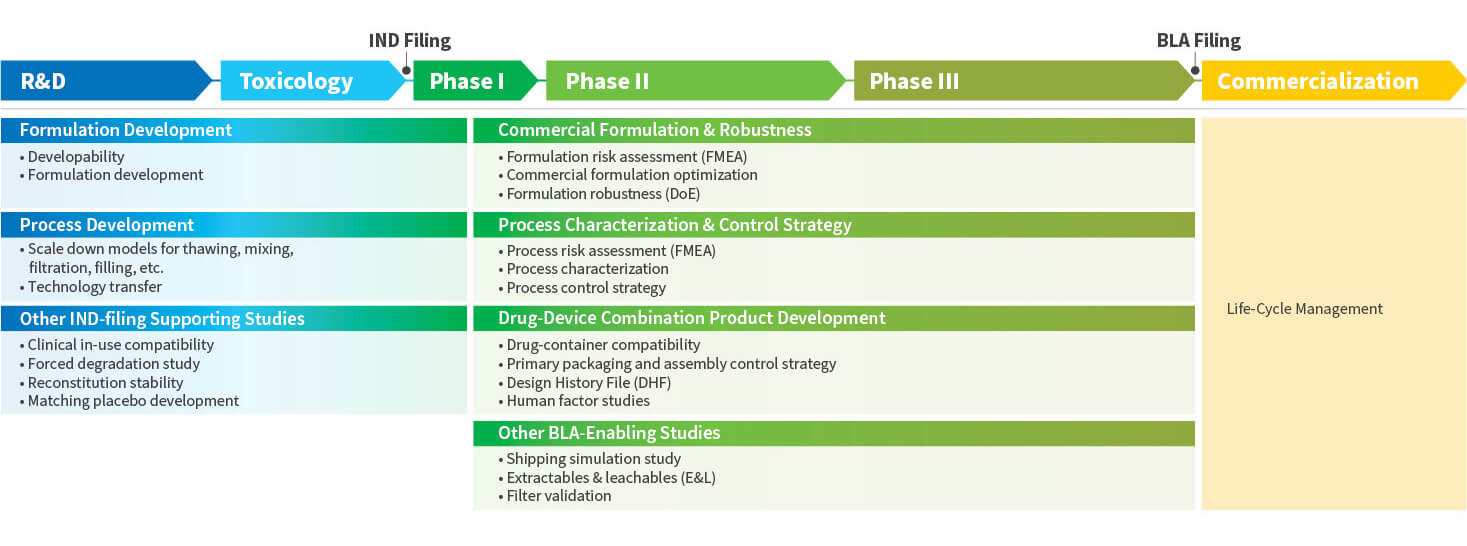
Drug Product Development
WuXi Vaccines provides industry-leading and state-of-the-art final vaccine and prophylactic antibody product development. We specialize in the development of clinical and commercial formulations and processes. Our capabilities include the development of liquid, frozen, lyophilized, emulsion, and suspension dosage forms and we have extensive experience with multiple container closure systems (CCS) that include vials and pre-filled syringes (PFS).
Program Features:
- High-concentration formulations of up to 200 mg/mL and as low as 1.5 ug/mL
- Experience in multivalent formulation development
- Integrated high-throughput and automation instrumentation to accelerate the path from development to BLA
- Development of liquid, lyophilization, frozen, emulsion, and suspension dosage forms
- Process development for standard/traditional or RTU vials and PFS
- End-to-end comprehensive capabilities from early-stage R&D formulation development through to late-stage final formulation lock and process characterization, qualification and validation activities prior to BLA filing
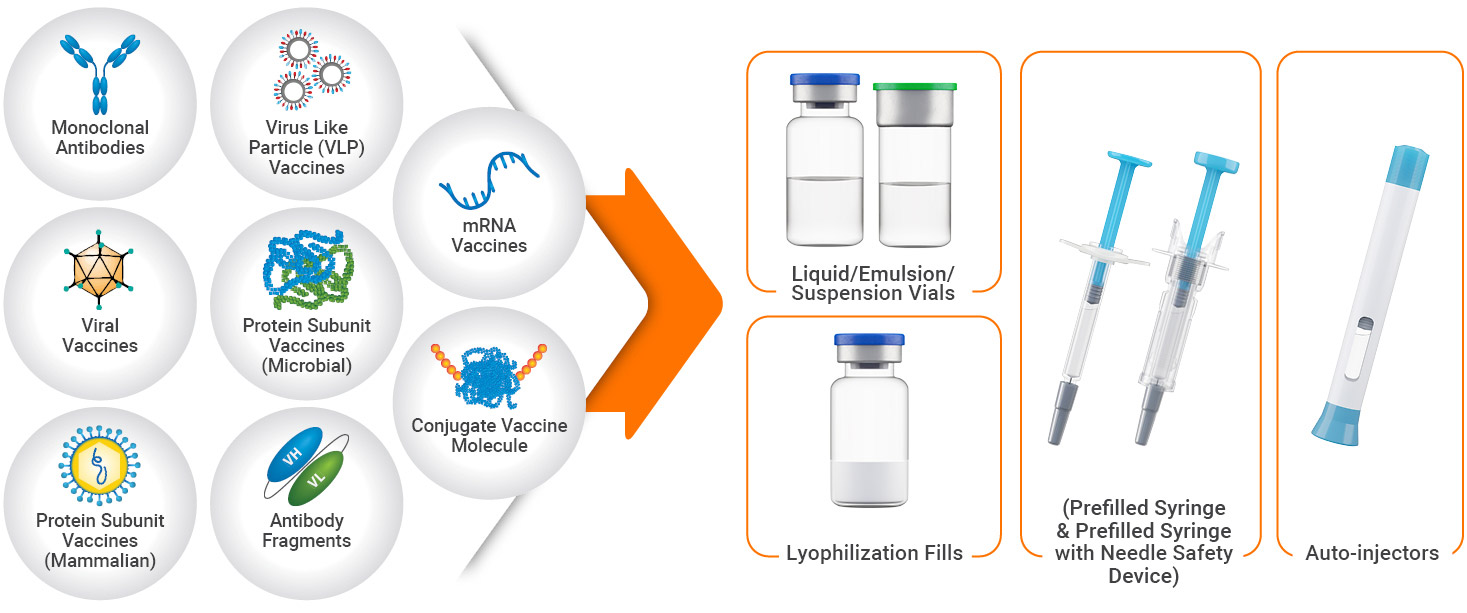
Starting with early-stage developability assessment and providing support throughout the entire formulation development life cycle, our services are suited to both large pharma corporations and early-stage companies. We have significant expertise in the development of the full range of modalities:
- Recombinant protein subunit vaccines
- Outer membrane vesicles (OMV) vaccines
- Viral vectored vaccines
- Live-attenuated viral vaccines
- Protein nanoparticles and Virus-like particle (VLP) vaccines
- Nucleic acids (e.g., DNA, mRNA/RNA, pDNA as raw material for mRNA or viral vaccines)
- Prophylactic antibodies
Specialized services include:
- Early-stage developability assessment for candidate evaluation
- Design of experiment (DOE) or one-factor-at-a-time (OFAT) experiments to identify the optimal formulation
- In-use compatibility studies to simulate and support clinical dose preparation, storage and administration in clinical setting
- Shipping simulation and validation
- Extractables and leachables (E&L) studies
- Forced degradation and stability studies
- High-throughput screening (HTS) using biophysical methods such as dynamic light scattering (DLS), differential scanning calorimetry (DSC), and differential scanning fluorimetry (DSF) among other advanced analytical equipment and methods
Regardless of your development phase, our scientists design the ideal formulation that can be adapted for the container closure system (CCS) of your choosing. Ready-to-sterilize (RTS) and ready-to-use (RTU) vials in multiple sizes are available for liquid, frozen, and lyophilized dosage forms. For clients looking for specialized drug delivery systems, prefilled syringes (with or without NSD) and autoinjectors are also available.
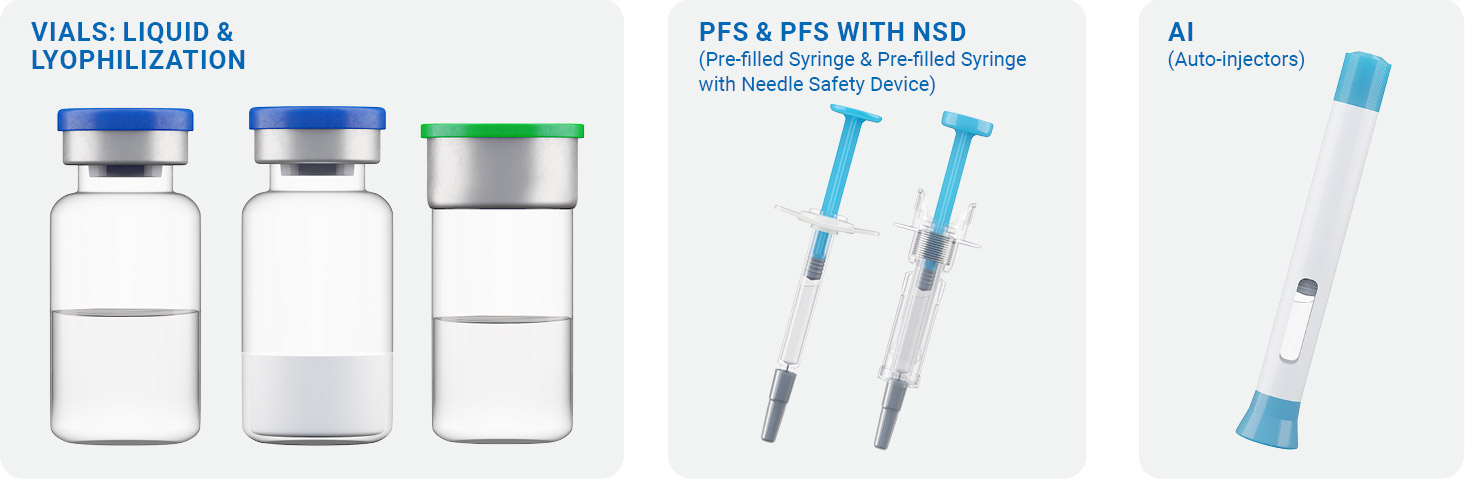
Container Closure Systems Features:
- Vials in 2R, 6R, 8R, 10R, 14R, 20R, and 50R sizes with RTS and RTU configurations
- Prefilled syringes (PFS) in 1 mL long, 1 mL standard, 2.25 mL, 3 mL syringe with staked needle or luer lock design
- PFS with passive needle safety device (NSD) options
- Autoinjector products optimized for improved user experience
- Pilot, clinical phase and large-scale commercial GMP production for all CCS types
To expedite your path to clinical or commercial vaccine manufacturing, our team of development experts support fill process development, scale-up and characterization using well established lab-scale models and full state-of-the-art analytical support.

Leveraging our advanced and automated fill line technologies, we offer a variety of process development and technology transfer services for process development for RABS (Restricted Access Barrier Systems), isolator-based filling lines, and fully-automated Vanrx systems including:
- Pilot scale (non-GMP) fill finish and lyophilization services for preclinical toxicology studies, non-GMP stability studies and scale-down process development
- End-to-end fill process development from bulk thaw through mixing, filtration, filling, stoppering and capping to final visual inspection
- Lyophilization cycle development and optimization
- Container and closure selection and integrity testing
Improved vaccine delivery solutions are becoming more vital to patient health and safety. Combination products such as prefilled syringes (PFS), PFS with needle safety devices (NSD) and autoinjectors offer greater convenience, operability and compliance to foster better patient outcomes.
WuXi Vaccines development teams offer the expertise and state-of-the-art equipment to design and develop the ideal delivery systems for your intended purpose and technical specifications. Our combination product development services assessment and evaluation includes:
- PFS functionality
- Silicone oil distribution
- Dimensional measurements
- Container closure integrity
- Automated PFS GMP filling lines
- Design Control
- Safety devices and autoinjectors assembly
Prefilled Syringe & Prefilled Syringe with Needle Safety Device Options
|
Activation Method |
Passive |
| Syringes Available
|
• 1 mL long, 1 mL standard, 2.25 mL, 3mL Syringes • Staked Needle & Leur Lock |
| Plunger Stoppers Available |
• 1 mL long & 1-3 mL plungers • Nest, RTP Bag |
| Filling Volume |
• 0.15 mL – 3 mL |
| Flange Type |
• Cut (CF), Round (RF), Small Round (SRF) • Extended Finger Flange • Plunger Rod |
| Customization Options |
• Color, Material • Plunger Rod & optional extended finger flanges |
Autoinjectors (AI)
WuXi Vaccines works with well-established syringe and autoinjector vendors for combination product developers. For automated injectors (AI), our specialized team of our highly-trained scientists can evaluate:
- Standard design optimized for user experience
- Fully customizable based on client specific schematics
- Design optimized through AI selection, assembly, and human factor considerations
Using SOP-driven procedures, our development teams for technology transfer works closely with our GMP manufacturing teams to ensure smooth and efficient technology transfer from late-stage development into GMP production.
After technology transfer from our development team to our GMP operations, WuXi Vaccines leverages its single-source process technology platforms and expertise to provide clients with efficient and cost-effective GMP manufacturing solutions. Our global GMP manufacturing facilities provide automated and isolator-based production under current Good Manufacturing Practice (cGMP) conditions as defined by the worldwide regulatory agencies and our quality systems have been routinely audited by our clients and have passed inspections by multiple regulatory agencies.
Throughout all stages of the final vaccine product development lifecycle, starting from the development of First-in-Human (FIH) formulations through to late-stage container closure selection, final formulation lock and process validation, our analytical development teams support your program by utilizing state-of-the-art equipment and methodologies to provide all services in-house and to expedite your timelines.
WuXi Vaccines End-to-End DP Development Program
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






