WuXi Biologics
Offering End-to-End Solutions
cGMP Manufacturing

Drug Substance (DS) Manufacturing
Implementing next-generation facility designs for multi-product facilities, a “scale-out” single-use bioreactor production scheme and continuous bioprocessing technologies, WuXi Vaccines has become a leading global vaccines supplier for our partners and clients. The success of our GMP operations is also due to our concept to commercialization vaccine development platform and excellence in project execution.
Each manufacturing facility shares the same quality system, and each site maintains dedicated in-house supporting functions such as QC testing labs, GMP warehouse, bio-waste and wastewater treatment, utilities (e.g., HVAC, WFI, Clean Steam, etc.), process automation systems (e.g., Delta V + OSI Pi Historian) and two independent power lines and back-up diesel generators.
Drug Product (DP) cGMP Manufacturing & Fill
We currently maintain drug product facilities capable of conducting liquid, lyophilized, emulsion and suspension fills at varying clinical and commercial scales from 800ml – 5L small scale to 20 million doses per year. We utilize a variety of container and closure system (CCS) configurations that include vials, prefilled syringes and other combination product CCS. All of our final vaccine product facilities provide RABS/isolator-based, fully-automated final product formulation and filling under Current Good Manufacturing Practice (cGMP) conditions as defined by the worldwide regulatory agencies, including the United States Food and Drug Administration (U.S. FDA), European Medicines Agency (EMA) and National Medical Products Association (NMPA). We have provided cGMP produced vaccines for clinical and commercial use in a variety of countries and regions.
The success of our GMP drug product operations is also due in part to our concept to commercialization drug development platform, extensive GMP drug substance manufacturing network, outstanding quality system and excellence in project execution.
Quality Control
- Area: ~1,000 m2, including dedicated Physiochemistry, Biochemistry, Bioassay and Microbiology Labs
- Personnel: 60% with Master’s degree or above
- GMP Compliance: Advanced instruments and testing platforms in compliant with China, USA, and EU cGMP requirements
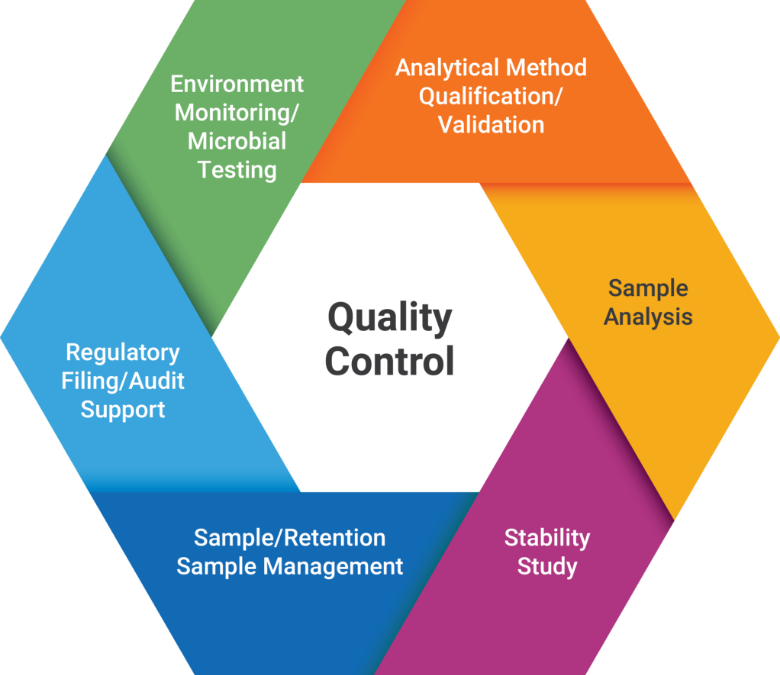
Testing capabilities include but not limited to:
- Identity: Peptide Mapping, Isoelectric Point, Spectral Analysis
- Compendia: Appearance, Color, Clarity, Visible Particles, Particulate Metter
- Quantity: Protein Concentration, Titer, PS20/80 Content
- Purity: UPLC & HPLC, Capillary Electrophoresi
- Impurity: Residual Protein A, Residual HCP, Residual DNA
- Bioassay: Cell-based and Binding ELISA Assay
- Safety: Bioburden, Bacterial Endotoxin, Sterility, CCIT
- Environment Monitoring: Water and Steam Systems, Process Gases, Clean Area Particles and Microorganisms
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?





