WuXi Biologics
Offering End-to-End Solutions
Analytical Development
At WuXi Vaccines, our analytical development teams facilitate our one-stop R&D endeavors in collaboration with our other discovery and CMC development laboratories. We offer comprehensive analytical services for a wide variety of vaccines (e.g., VLP and recombinant protein subunit) or prophylactic antibodies.
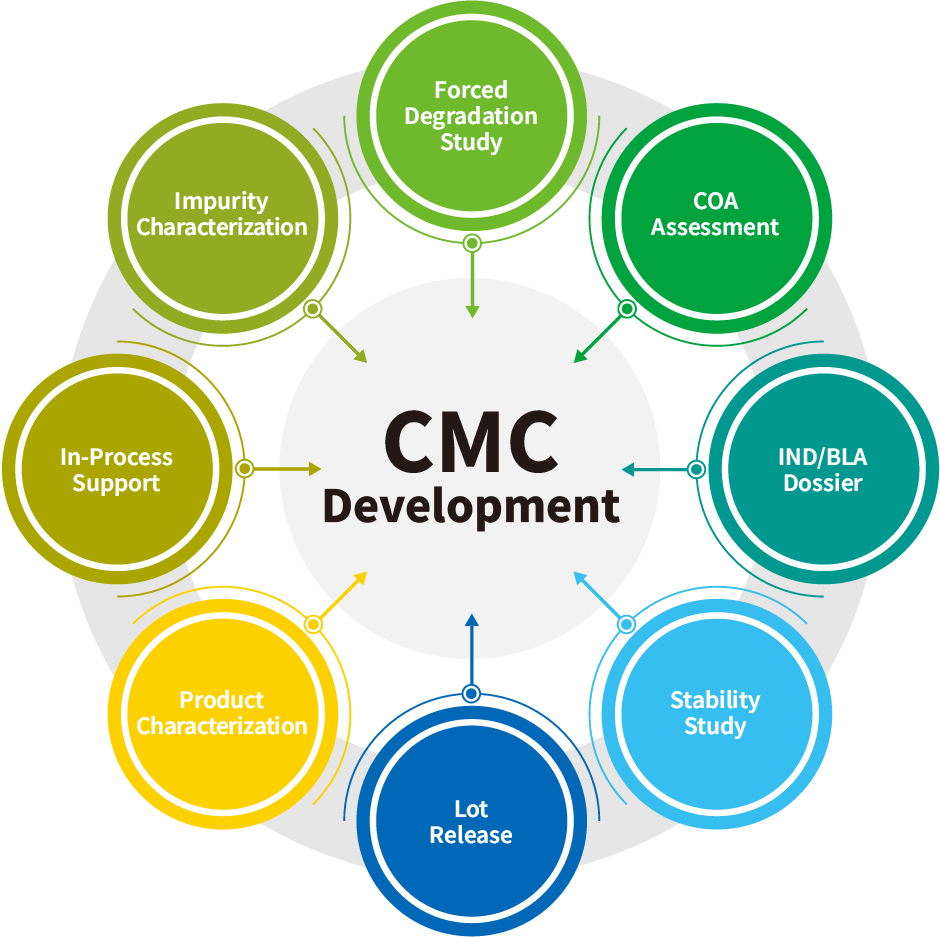
Our extensive testing services support developability studies, in-process analysis, cell line, process development, formulation development, product characterization, lot release, stability studies and IND/BLA/MAA/EUA dossier preparation.
Our vast repertoire of analytical techniques provides the full range of methods required for biomolecular analysis. Over 1,000 established methods including compendial, physicochemical, biochemical, biophysical and biological assays (bioassays) supported by our experienced teams are available. From new vaccine and antibody discovery to regulatory filing, we manage a method’s lifecycle from development to qualification and validation and transfer to quality control or other departments and provide investigation and troubleshooting support as needed. Equipped with cutting-edge instrumentation and first-class talent, we provide systematic solutions for integrated projects while catering to special analytical needs in the form of standalone projects.
Comprehensive Analytical Services to Support CMC Development
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






