WuXi Biologics
Offering End-to-End Solutions
Downstream Process Development
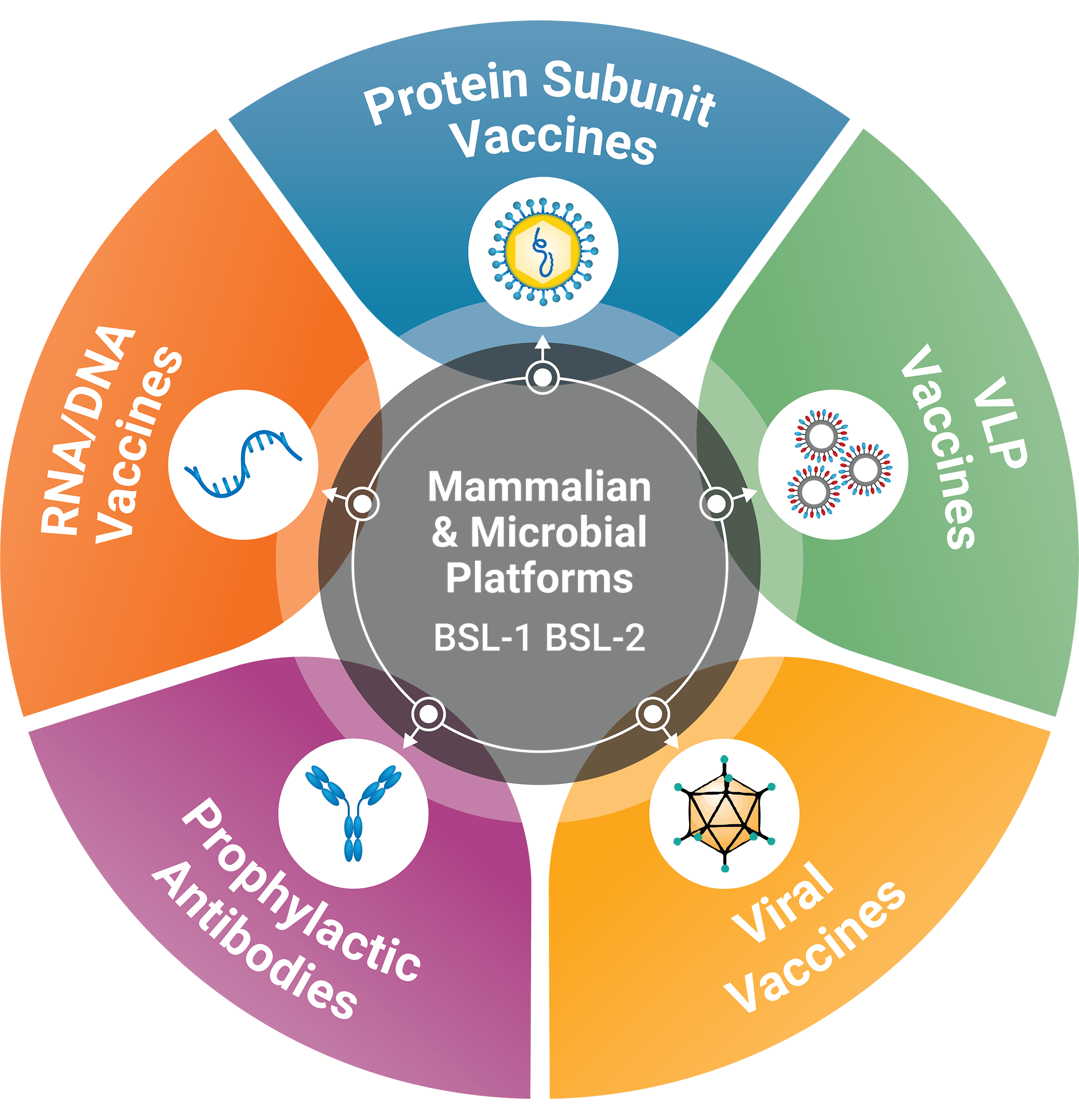
Our downstream process development (DSPD) team applies state-of-the-art technology to purification process development, process scale-up, process characterization, and technology transfer to cGMP production. DSDP has extensive experience developing purification schemes for a wide-variety of prophylactic antibodies and vaccines, including VLP and recombinant protein subunit approaches.
Downstream Process Platform
-
- The downstream process development (DSPD) team can develop cGMP purification processes for a wide range of products to meet client needs. An array of chromatographic resins, including affinity, ion exchange, hydrophobic interaction, hydroxyapatite, mix-mode can be screened. These downstream process development activities can be completed within in 12–24 weeks.
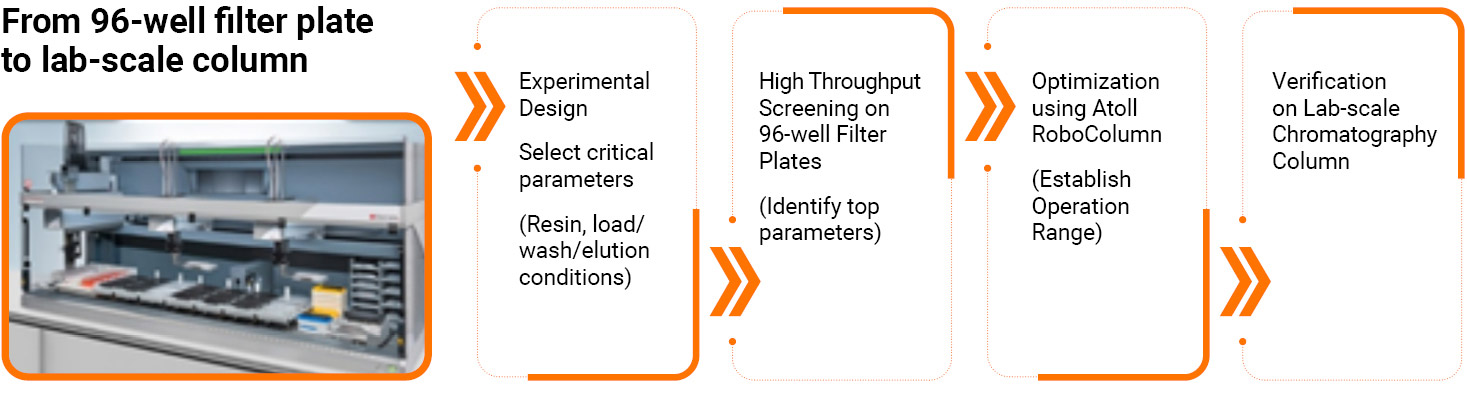
High Throughput Process Development Platform
Continuous Processing
-
-
- Our team of DSPD experts can establish new purification schemes or convert batch processes to a continuous process. We can work with our upstream cell culture process development team to integrate downstream continuous process operations into one continuous processing platform.
-
- Convert batch process to continuous process in 2 weeks
-
- Reduce the Protein A resin cost by up to 90%
-
- Increase downstream productivity by 100%
-
- Increase downstream capacity by 1,000%
-
Continuous Processing
To the support our client’s integrated development programs, we offer customized standalone services to either develop entire purification schemes or single unit step operations or processes.
Purification Support:
-
- Purification to support clone selection and cell culture development
- DS generation to support analytical and formulation development activities
Early Phase Downstream Process Development:
-
- Bench-scale process development
- Process scale-up in non-GMP pilot plant
- Technology transfer for GMP production
- Viral clearance studies (if required)
Late Phase Downstream Process Optimization:
-
- Process optimization
- Failure Modes and Effects Analysis (FMEA)
- Scale-down model qualification for process characterization
- Process characterization
- Late phase viral clearance study (if required)
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






