WuXi Biologics
Offering End-to-End Solutions
mRNA Vaccines
Messenger RNA Therapeutics and Vaccines
WuXi Vaccines offers extensive capabilities and capacity in the development and GMP manufacturing of messenger (mRNA) vaccines. Our highly trained and expert team offers end-to-end services, starting from plasmid DNA (pDNA) manufacturing to the production and release of the final mRNA vaccine product under GMP conditions. We have dedicated GMP facilities, including a microbial-based manufacturing facility equipped with fermenters of up to 300 L that scale for pDNA production. For mRNA IVT, reactions up to 50 L along with multiple vessels and purification equipment cover a wide range of reaction volumes for mRNA transcription and purification.
Our DP manufacturing capabilities include state-of-the-art mRNA-LNP production systems and robotic aseptic filling systems. All our services are built on our exceptional in-house CMC development and manufacturing capabilities and expertise, driven by a commitment to executional excellence and adherence to world-class quality systems.
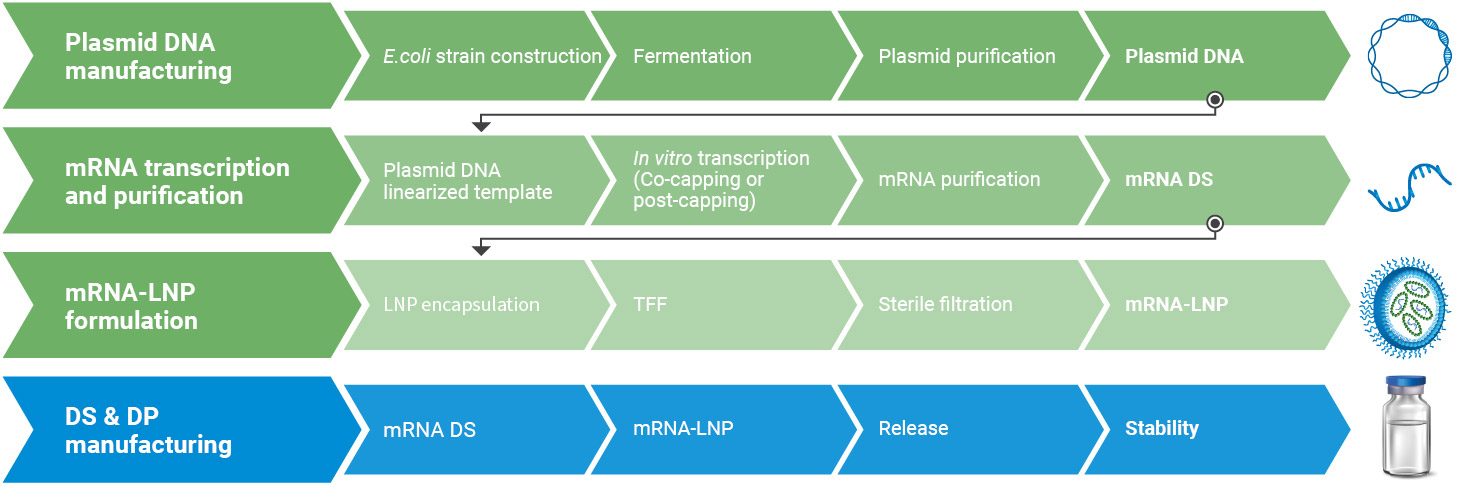
Integrated end-to-end services from sequence to IND
Key services include:
- Sequence optimization
- Process development
-
- pDNA: E. coli fermentation, cell lysis and purification
- mRNA: In vitro f transcription (IVT), including co-capping or post-capping options, and purification
- LNP: Encapsulation, ultrafiltration/diafiltration, and fill and finish
- Technical transfer and scale-up
- cGMP manufacturing: DS (mRNA) and DP (mRNA-LNP)
- Comprehensive analytical development and QC testing
- Full CMC and regulatory support
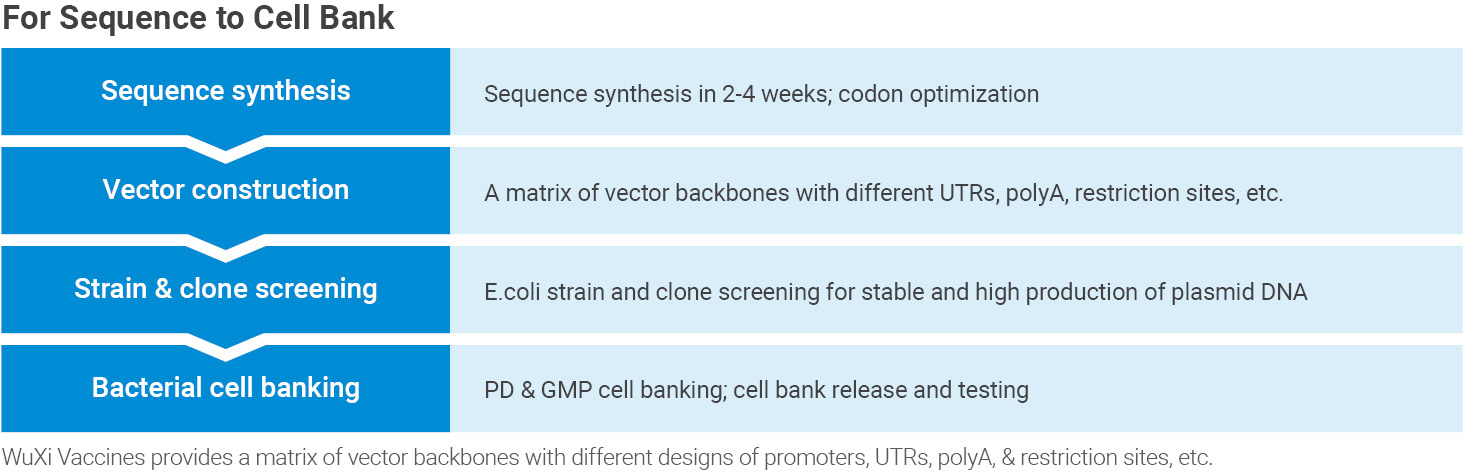
Bulk Vaccine Process Development and Manufacturing
Our GMP offerings include a manufacturing facility with a fermenter that scales up to 300 L for pDNA production. Various vessel volumes and purification equipment support mRNA transcription and purification.
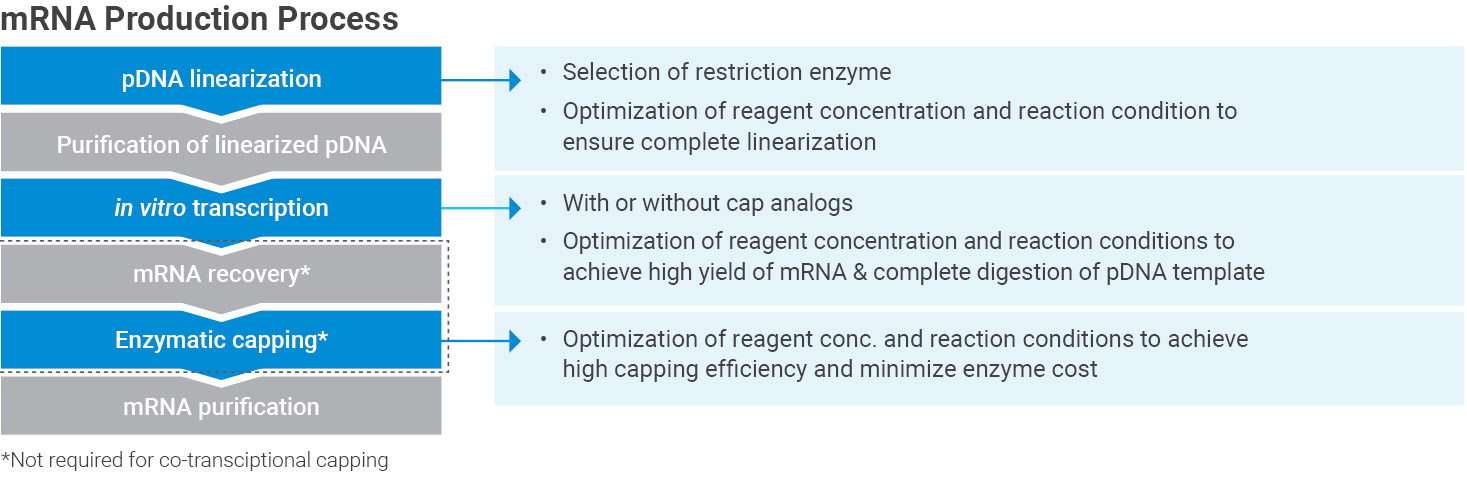
Final Vaccine Drug Product Process Development and Manufacturing
Our manufacturing service includes state-of-the-art mRNA-LNP production systems (e.g. Microfluidic and T-Junction mixing techniques) as well as robotic aseptic filling systems.
RNA Vaccine Product Development
- Formulation development, process development and technology transfer
- Lyophilization cycle development
- Stability, stress study, CCIT, clinical in-use study
- 25 µL to 10 L scale (feasibility / pilot)
- Extensive mRNA-LNP DP characterization techniques: particle size, PDI, encapsulation rate, potency, etc.
RNA Vaccine Final Product Manufacturing
- RABS (Restricted Access Barrier Systems) , isolator-based filling lines and two segregated Vanrx SA25 Robotic filling lines
- Microfluidic and T-junction encapsulation processes
- Aseptic formulation isolator
- Single-use systems
- Ready-to-use container-closure systems (vials and pre-filled syringes)
Analytical Science and Quality Control
Our dedicated team of highly trained and experienced analytical and QC professionals, allows us to offer:
- Integrated labs that support diverse mRNA modalities
- Methods development, transfer and qualification/validation
- Product biochemical, biophysical, and biological characterization, and comparability analysis
- Comprehensive analytical capabilities for full CMC packages that enable IND/BLA filings
- Tox/Clinical lot DS/DP release and stability testing
- Reference standard generation, qualification, characterization, storage, and life-time management
- Analytical investigation; troubleshooting of GMP manufacturing related issues
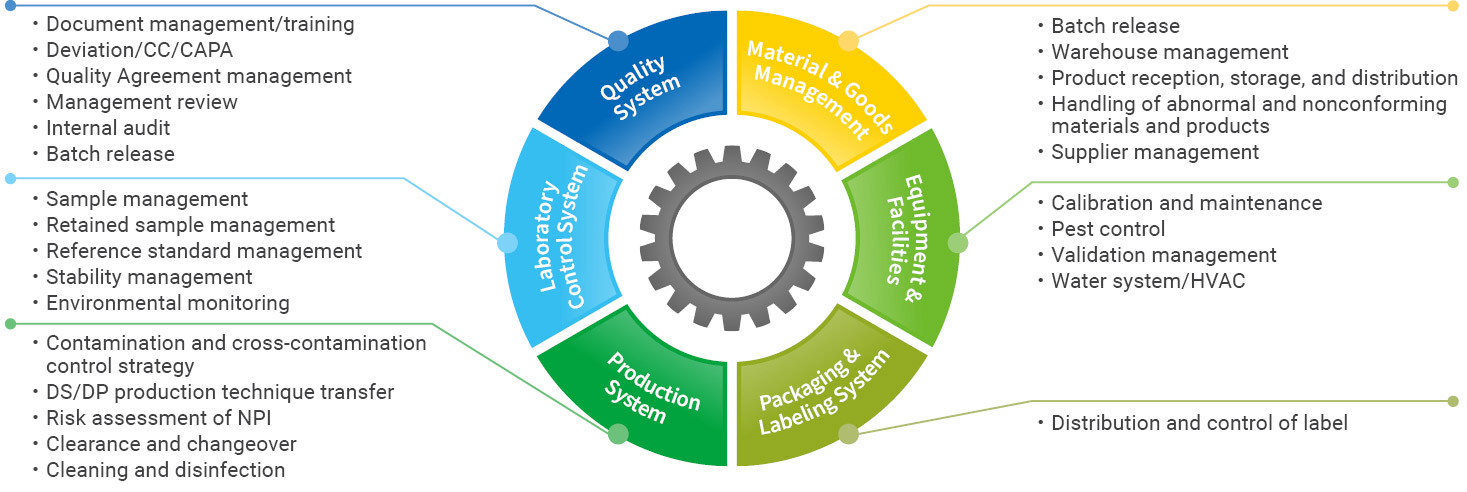
Our established global quality system, quality control and operational systems meet or exceed worldwide regulatory standards from the U.S. FDA, EMA, NMPA and other major regulatory agencies. Our commitment to quality is ingrained in our company culture and our employees. We have harmonized our quality assurance (QA) and quality management system across all sites around the world for the clinical or commercial production of vaccines drug substance and drug product.
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






