WuXi Biologics
Offering End-to-End Solutions
Modalities
Overview
WuXi Vaccines provides comprehensive CMC development and GMP manufacturing services for a wide range of modalities produced from microbial production systems. As a leading contract development and manufacturing organization (CDMO), we have established world-class BSL-1 & BSL-2 facilities and a highly trained team of experts to provide end-to-end services from strain development to regulatory filing and beyond. Leveraging these technical capabilities alongside our project management systems enables us to expedite product development and bring critical vaccines into the clinic and onto the market.
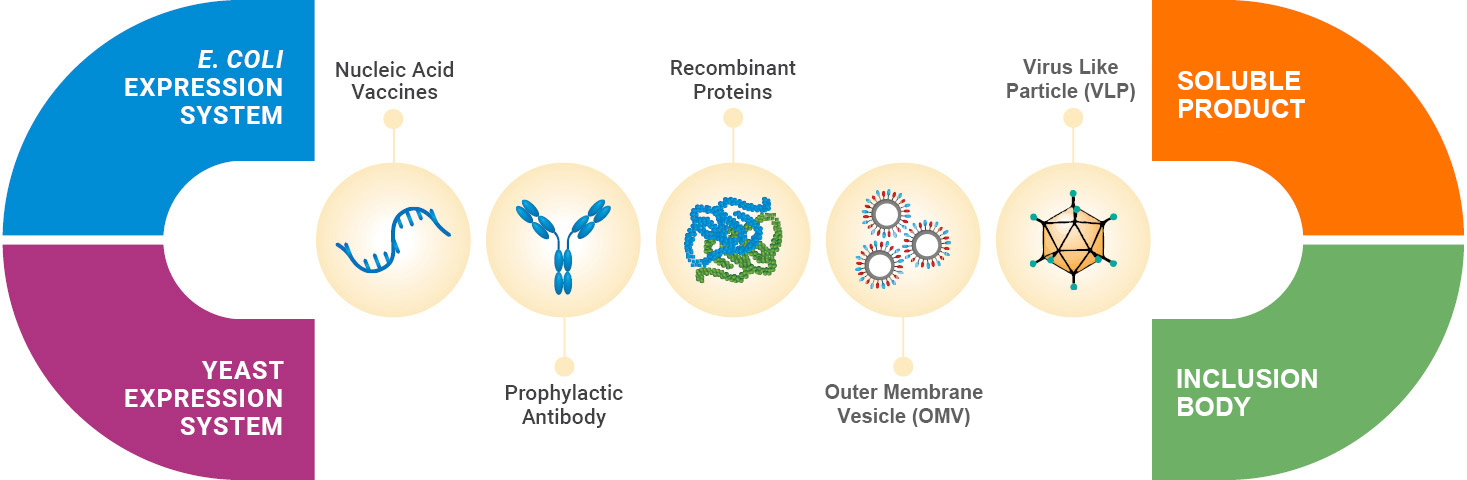
Comprehensive Range of Supported Product Modalities
We support an array of prophylactic products produced via microbial fermentation which include recombinant proteins (e.g., subunit vaccines), virus-like particle (VLP), outer membrane vesicle (OMV), or nucleic acid-based vaccines. Our support includes production of GMP-grade plasmid DNA (pDNA) that might be required as vaccines or to support mRNA-based vaccine programs. Our integrated service offerings include comprehensive strain development; process development; analytical, formulation, and drug product development, GMP manufacturing for drug substance (DS) and drug product (DP).
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






