WuXi Biologics
Offering End-to-End Solutions
cGMP Manufacturing

Drug Substance (DS) Manufacturing
Implementing next-generation facility designs for multi-product facilities, a “scale-out” single-use bioreactor production scheme and continuous bioprocessing technologies, WuXi Vaccines has become a leading global vaccines and prophylactic antibodies supplier for our partners and clients. The success of our GMP operations is also due to our concept to commercialization vaccine and prophylactic antibody development platform and excellence in project execution.
Each manufacturing facility shares the same quality system, and each site maintains dedicated in-house supporting functions such as QC testing labs, GMP warehouse, biowaste and wastewater treatment, utilities (e.g., HVAC, WFI, Clean Steam, etc.), process automation systems (e.g., Delta V + OSI Pi Historian) and two independent power lines and back-up diesel generators.
Drug Product cGMP Manufacturing
We maintain DP facilities capable of conducting liquid, lyophilized, emulsion, and suspension fills at varying clinical and commercial scales from 1–5 L DS scale to 20 million doses per year. We use a variety of container and closure system (CCS) configurations including vials, prefilled syringes, and other combination product CCSs.
All our final vaccine and prophylactic antibody product facilities provide oRABS/isolator-based, automated final product formulation and filling under cGMP conditions as defined by the worldwide regulatory agencies, including the Food and Drug Administration (FDA), European Medicines Agency (EMA), and National Medical Products Association (NMPA). We have provided cGMP produced vaccines and prophylactic antibodies for clinical and commercial use in a variety of countries and regions.
The success of our cGMP DP operations is also due to our concept to commercialization development platform, extensive cGMP DS manufacturing network, outstanding quality system, and excellence in project execution.
Quality Control
- Area: ~1,900 m2, including dedicated Physiochemistry, Biochemistry, Bioassay and Microbiology Labs
- Personnel: 60% with Master’s degree or above
- GMP Compliance: Advanced instruments and testing platforms in compliant with China, USA, and EU cGMP requirements
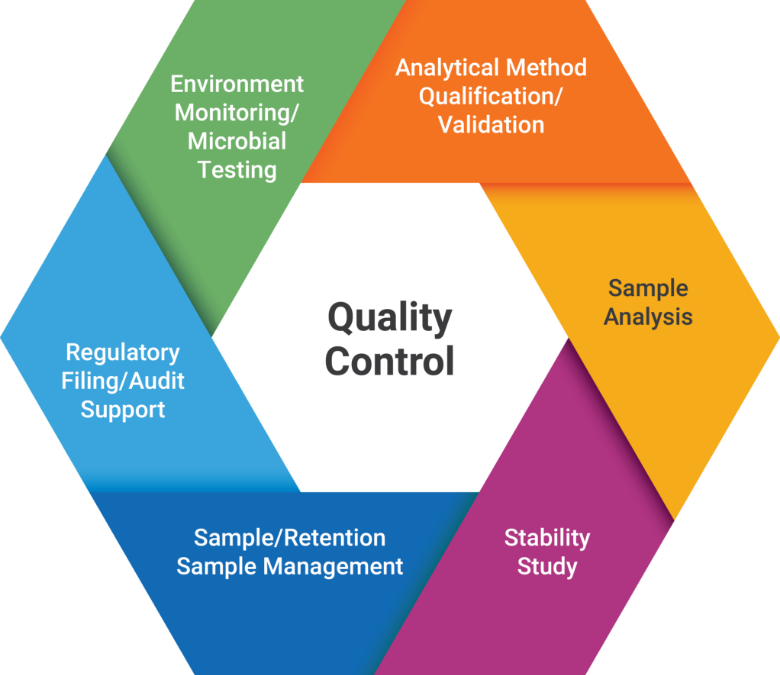
Testing capabilities include but not limited to:
- Identity: Peptide Mapping, Isoelectric Point, Spectral Analysis
- Compendia: Appearance, Color, Clarity, Visible Particles, Particulate Metter
- Quantity: Protein Concentration, Titer, PS20/80 Content
- Purity: UPLC & HPLC, Capillary Electrophoresis
- Impurity: Residual Protein A, Residual HCP, Residual DNA
- Bioassay: Cell-based and Binding ELISA Assay
- Safety: Bioburden, Bacterial Endotoxin, Sterility, CCIT
- Environment Monitoring: Water and Steam Systems, Process Gases, Clean Area Particles, and Microorganisms
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






