WuXi Biologics
Offering End-to-End Solutions
Late Stage Development and Commercialization
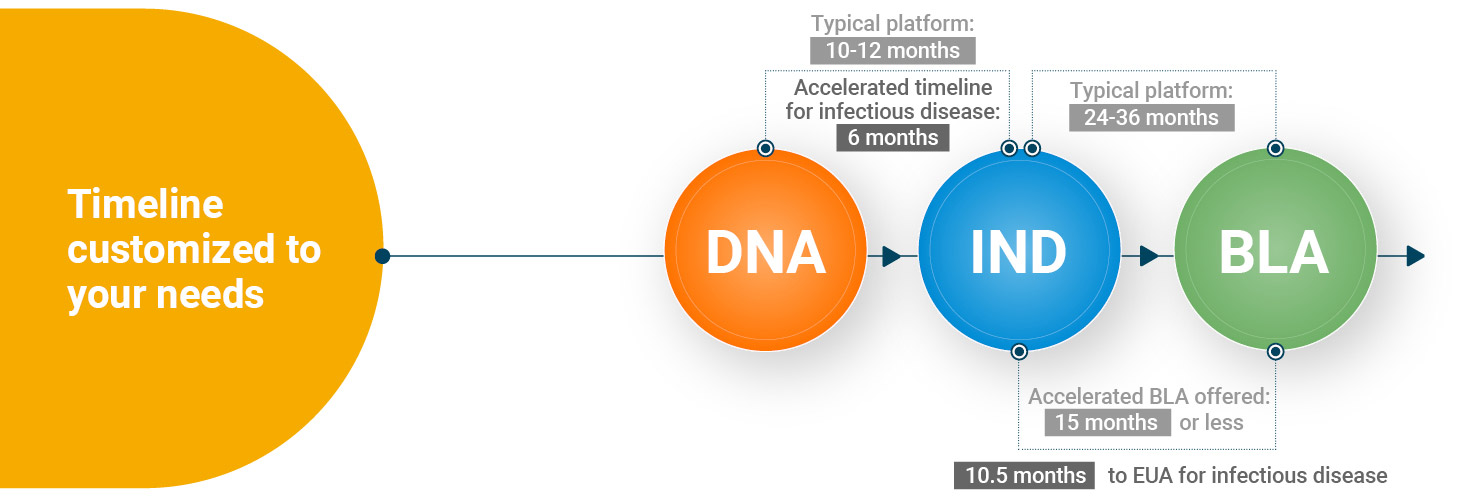
WuXi Vaccines provides single-source, high-quality late-phase development and manufacturing services for companies looking to bring vaccines and prophylactic antibodies to the market. Through our highly vetted development platforms designed to accelerate your product through all aspects of the late phase process, assay and formulation development, optimization, characterization, and validation, we ready your projects for BLA filing in as little as 14 months. Additionally, our extensive global vaccine and prophylactic antibody supply network provides the necessary GMP manufacturing capacities to supply commercialized products on a worldwide scale once you are ready to move beyond the BLA phase.

Essential Program Activities:
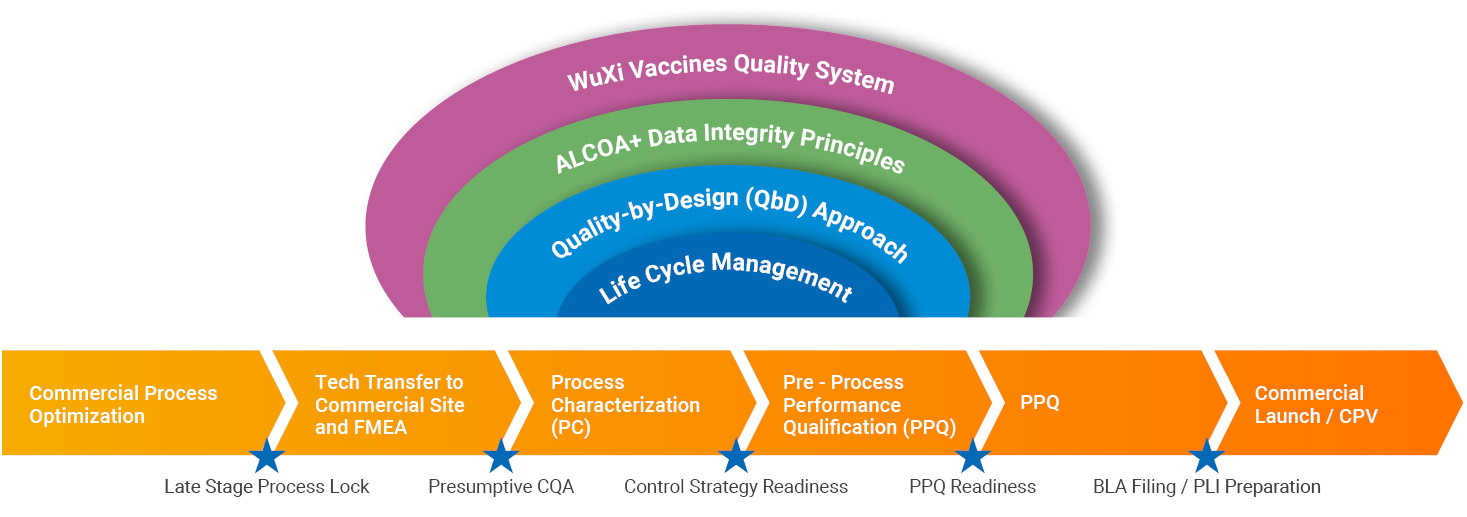
We use life cycle management and quality-by-design principles outlined in the ICH guidelines as the fundamental backbone to all our process development (PD), process characterization (PC) and process performance qualification (PPQ) activities and couple that with ALCOA+ data integrity principles to provide you with the highest quality late-phase development program.
Process Characterization
WuXi Vaccines’ late-stage development program adheres to rigorous scientific standards and a detailed methodology to meet the eventual regulatory scrutiny upon BLA filing. To ensure your product reaches its potential in terms of safety and efficacy, we lay down the foundation for quality early in the commercial process development and optimization phase.
- Optimize late phase process
- Tech transfer to commercial facility
- Perform initial Failure Mode and Effects Analysis (FMEA)
- Define presumptive CQAs
- Build scale down model and PC
- Conduct final FMEA, control strategy, CQA confirmation before PPQ preparation
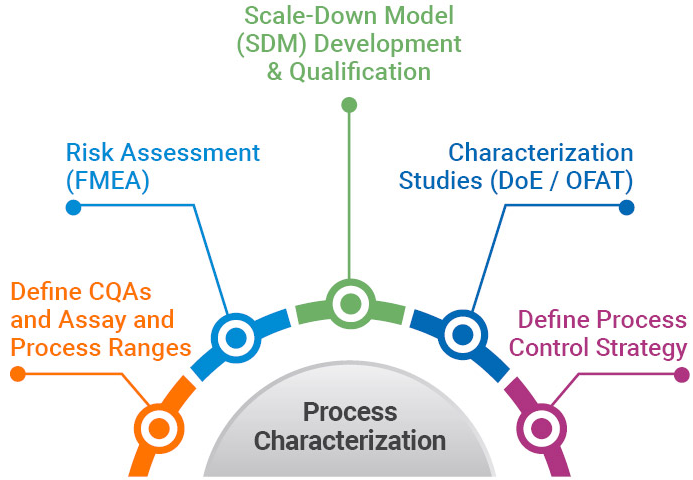
By identifying the presumptive critical quality attributes (CQAs) that affect potency, safety, and product quality, we perform process characterization to better understand and confirm the process and refine CQAs. Key process parameters must be defined by failure mode and effects analysis (FMEA). Through design of experiment (DoE) and one-factor-at-a-time (OFAT) methods, we characterize each process step using qualified scaled-down models. A final FMEA is then conducted to fully understand and confirm CQAs and define the control strategy, ensuring readiness for the process performance qualification (PPQ).
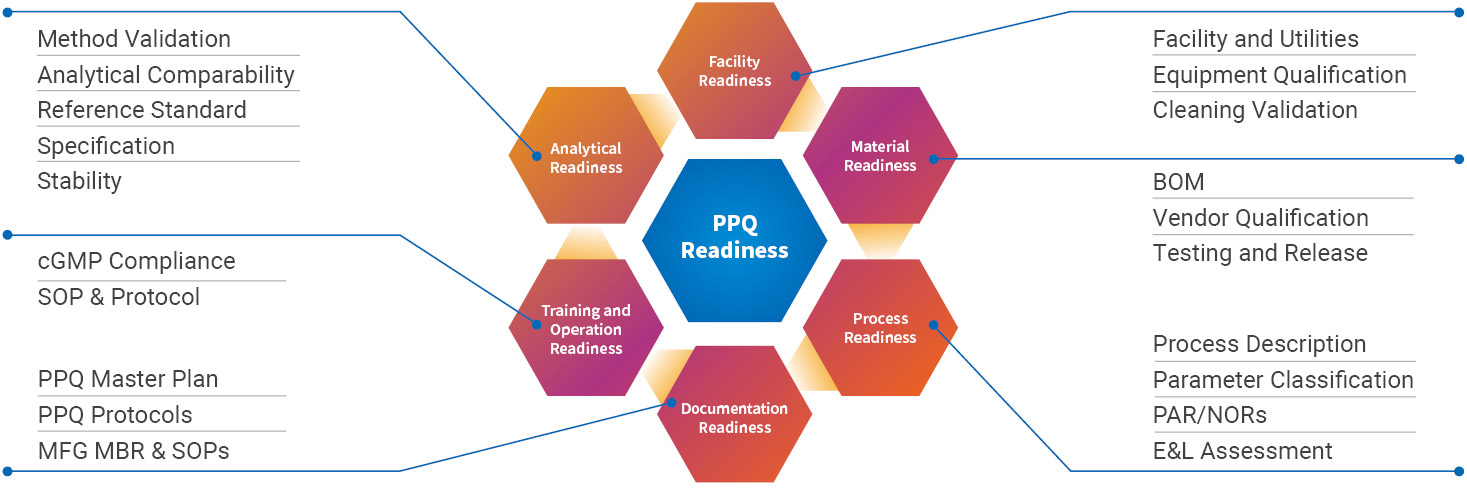
Process performance qualification (PPQ) demonstrates the validity of the process design and the suitability of the process control strategy at the commercial manufacturing scale. All critical aspects for PPQ readiness use our extensive in-house qualification and validation programs and enable a right-first-time approach.
- Process description and control strategy are ready with parameter classification, defined proven acceptable ranges (PARs), and normal operating ranges (NORs).
- Analytical methods validation for all in-process control and release testing with specification, reference standard, comparability and stability programs are in place.
- All raw material and excipients testing follow ICH guidelines and the bill of materials (BOM) and testing strategy are defined along with materials are ready and released.
- Facility readiness includes all facilities, utilities, instruments, and equipment qualification and calibration.
- Documentation readiness spans all PPQ plans and study protocols, manufacturing batch records and SOPs, and AMPs.
- Training of operators and the development of standard operating procedures (SOPs) ensures operation readiness and cGMP compliance.
All critical aspects of PPQ readiness using our extensive in-house qualification and validation programs enable a right-first-time approach including our comprehensive raw materials control system that has passed 30 global regulatory agency audits.
Our integrated approach includes myriad specialized capabilities designed to minimize risk and expedite your timeline to BLA filing.
|
|
To provide you with a global supply network, WuXi Vaccines operates multiple state-of-the-art DP cGMP manufacturing facilities across five countries. We maintain fill and finish facilities capable of conducting liquid or lyophilized, emulsion or suspension fills at varying clinical and commercial scales and utilizing a variety of container and closure system (CCS) configurations that include vials, prefilled syringes, and other combination product CCSs.
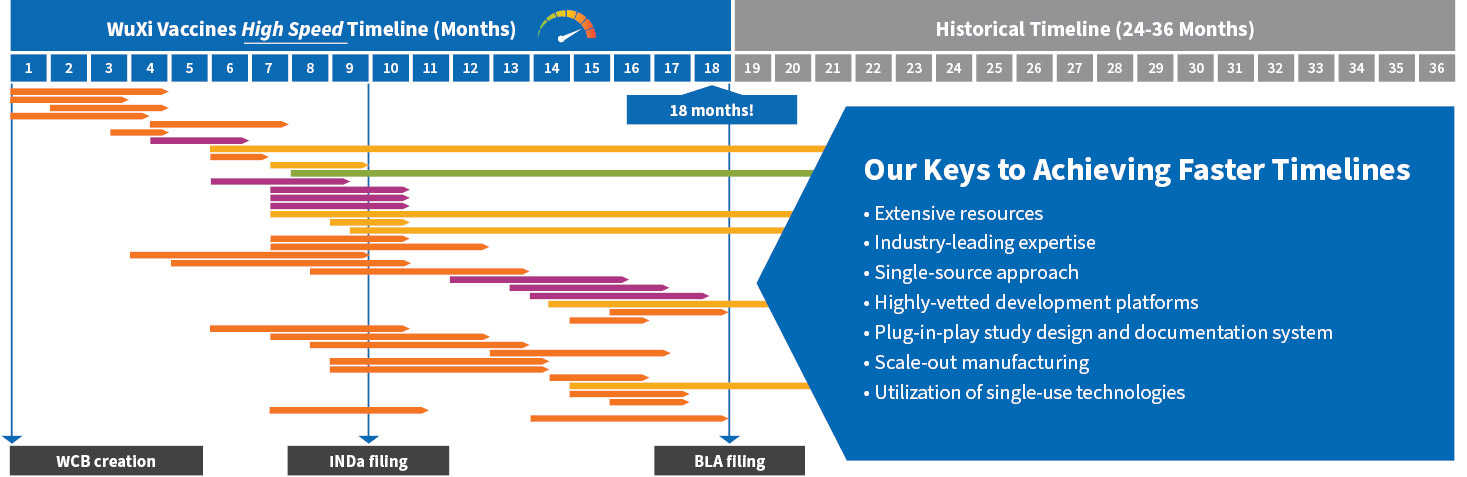
Our Keys to Achieving Faster Timelines
With state-of-the-art equipment and facilities at our disposal along with hundreds of industry veterans each with 10–30 years of experience, our highly trained scientists leverage extensive resources and industry-leading expertise to perform all program activities at accelerated speeds. Through our single-source approach and global manufacturing capacity, customers benefit from having no project handovers and a single project management system.
A plug-in-play study design and documentation system along with our deep process understanding allows us to provide a more efficient project start and in parallel, use our highly vetted development platforms to execute risk-based activities. Lastly, we take a scale-out manufacturing philosophy and employ single-use technologies to reduce scale-up risk in cell culture processes and cleaning validation workload.
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






