WuXi Biologics
Offering End-to-End Solutions
Viral Vectors for Vaccines
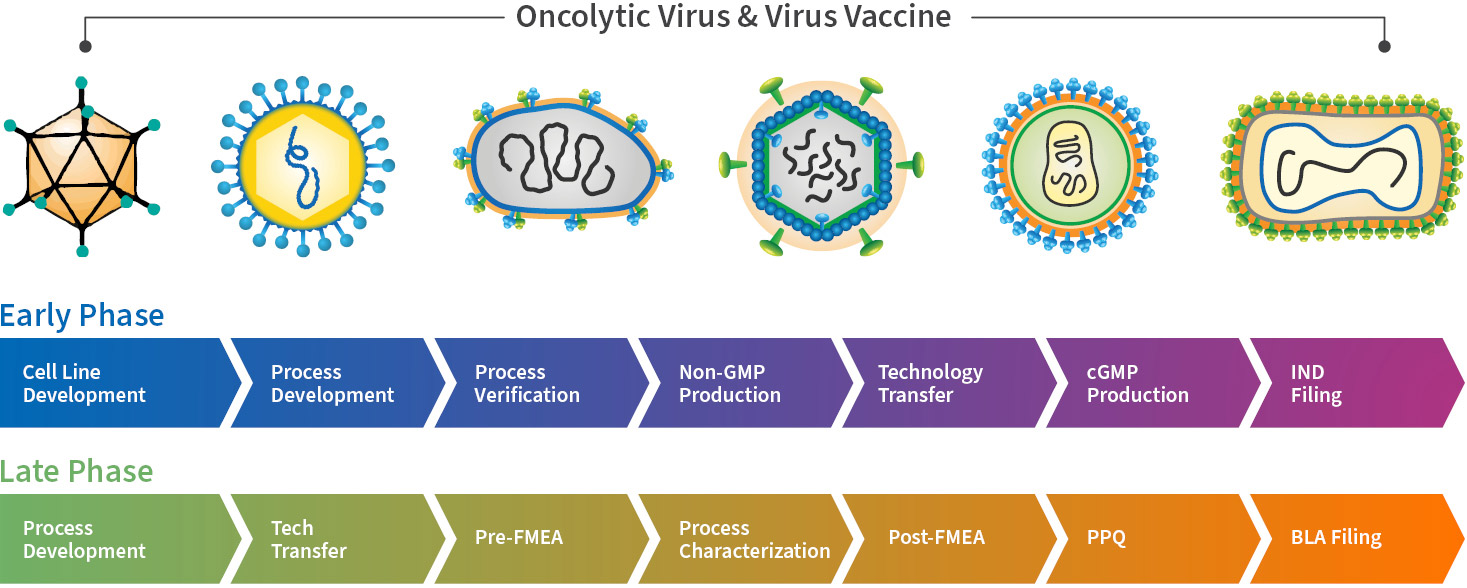
|
Virus Type
|
In-house Producer Cell*
|
CB/VS Banking
|
CB/VS Testing**
|
PD
|
DS and DP MFG
|
Lot Release and Stability
|
Regulatory Support
|
|
AdV
|
√
|
√
|
√
|
√
|
√
|
√
|
√
|
|
HSV
|
√
|
√
|
√
|
√
|
√
|
√
|
√
|
|
VSV
|
√
|
√
|
√
|
√
|
√
|
√
|
√
|
|
Vaccinia Virus
|
√
|
√
|
√
|
√
|
√
|
√
|
√
|
|
MV
|
√
|
√
|
√
|
√
|
√
|
√
|
√
|
| * In house producer cell under development at WuXi Vaccines ** Further information may be required for the cell line/virus seed testing capabilities and timeline WuXi Vaccines also provides high-quality and GMP-grade plasmid DNA production services to support viral vaccine development and manufacturing. Please refer to the Microbial Platform section for our capabilities and capacities for plasmid DNA production. |
|||||||
Notice:
You are leaving WuXi Biologics Website, after which our Privacy Notice will not apply. Please keep it in mind the protection of your privacy. Are you willing to proceed?






